Cara Therapeutics IncNASDAQ:CARANASDAQ FSI:D. Deficient (Issuer failed to meet continued listing requirements)
- LAST PRICE0.2878
- TODAY'S CHANGE (%)-0.0001 (-0.0521%)
- Bid / Lots0.2816/ 4
- Ask / Lots0.2898/ 15
- Open / Previous Close0.2879 / 0.2879
- Day Range
- 52 Week Range
- Volume29,322
Search Criteria
Filter search criteria using below inputs
Click on magnifying glass icon to search
| Company | Country | Symbol |
|---|
- TSX Comp
- TSX Venture
- DJIA
- S&P 500
- NASDAQ

| Time | Volume | CARA |
|---|---|---|
| 09:32 ET | 2422 | 0.2875 |
| 09:34 ET | 500 | 0.2875 |
| 09:39 ET | 1330 | 0.2893 |
| 09:41 ET | 2893 | 0.2832 |
| 09:43 ET | 17080 | 0.2899 |
| 09:54 ET | 1455 | 0.28001 |
| 09:59 ET | 398 | 0.284376 |
| 10:08 ET | 1095 | 0.2898 |
| 10:10 ET | 593 | 0.2898 |
| 10:15 ET | 500 | 0.28775 |
| Company sortable | Market Cap sortable | P/E Ratio (TTM) sortable | EPS Growth (5yr) sortable |
|---|---|---|---|
Cara Therapeutics Inc | 15.8M | -0.1x | --- |
Turnstone Biologics Corp | 12.9M | -0.2x | --- |
SQZ Biotechnologies Co | 1.0M | 0.0x | --- |
Viaderma Inc | 6.7M | 0.0x | --- |
ImmuCell Corp | 28.3M | -7.0x | --- |
Eiger BioPharmaceuticals Inc | 12.6M | -0.2x | --- |
Company Information
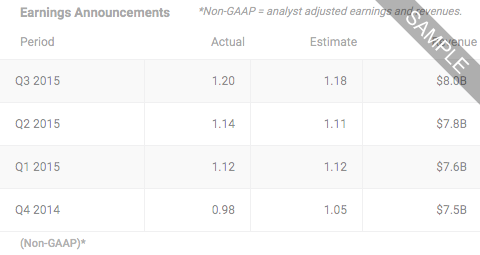
Cara Therapeutics, Inc. is a development-stage biopharmaceutical company. The Company is focused on treatment paradigm to improve the lives of patients suffering from chronic pruritus. It is developing an oral formulation of difelikefalin, a selective, predominantly peripherally acting, non-scheduled Kappa opioid receptor agonist, for the treatment of chronic neuropathic pruritus associated with Notalgia Paresthetica (NP), a common, underdiagnosed neuropathy affecting the upper back. It is conducting Phase II/III Clinical Program in NP. The program is comprised of two studies - KOURAGE 1 and KOURAGE 2, which are double-blind, placebo-controlled, eight-week studies with patients allowed to roll-over into open-label 52-week extensions. It has developed an IV formulation of difelikefalin, for the treatment of moderate-to-severe pruritus associated with advanced chronic kidney disease in adults undergoing hemodialysis. The IV formulation is out-licensed worldwide.
Contact Information
- Headquarters
- 400 Atlantic Street, Suite 500STAMFORD, CT, United States 06901
- Phone
- 203-406-3700
- Fax
- 203-567-1510
Executives
- Independent Chairman of the Board
- Martin Vogelbaum
- President, Chief Executive Officer, Director
- Christopher Posner
- Chief Financial Officer
- Ryan Maynard
- Chief Compliance Officer, General Counsel, Secretary
- Scott Terrillion
- Director
- Helen Boudreau
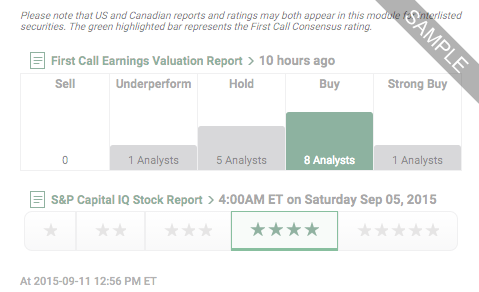
Our Ratings feature offers company-specific research ratings from providers such as First Call, S&P and Argus.
Open a New Account, or Login if you're a client.
You have access to a comprehensive selection of independent research reports from providers such as TD Securities, S&P, INK, and Argus.
Open a New Account, or Login if you're a client.
Market Cap | $15.8M |
|---|---|
Revenue (TTM) | $11.0M |
Shares Outstanding | 54.8M |
Dividend Yield | 0.00% |
Annual Dividend Rate | --- |
Ex-Dividend Date | 01-01-01 |
Pay Date | 01-01-01 |
Beta | 0.70 |
EPS | $-2.04 |
Book Value | $1.05 |
P/E Ratio | -0.1x |
Price/Sales (TTM) | 1.4 |
Price/Cash Flow (TTM) | --- |
Operating Margin | -1,000.06% |
Our Calendar feature allows you to view a wide selection of market and company events, including earnings, dividends, splits, rating changes, guidance, and more. Access past, current and future events in WebBroker.
Open a New Account, or Login if you're a client.