Passage Bio IncNASDAQ:PASGNASDAQ FSI:D. Deficient (Issuer failed to meet continued listing requirements)
- LAST PRICE0.6150
- TODAY'S CHANGE (%)0.0105 (1.7370%)
- Bid / Lots0.6030/ 4
- Ask / Lots0.6150/ 29
- Open / Previous Close0.6000 / 0.6045
- Day Range
- 52 Week Range
- Volume53,155
Search Criteria
Filter search criteria using below inputs
Click on magnifying glass icon to search
| Company | Country | Symbol |
|---|
- TSX Comp
- TSX Venture
- DJIA
- S&P 500
- NASDAQ

| Time | Volume | PASG |
|---|---|---|
| 09:32 ET | 4468 | 0.6 |
| 09:33 ET | 250 | 0.619 |
| 09:48 ET | 1800 | 0.6112 |
| 10:00 ET | 1200 | 0.6112 |
| 10:04 ET | 100 | 0.629 |
| 10:22 ET | 100 | 0.62 |
| 10:26 ET | 100 | 0.619 |
| 10:27 ET | 100 | 0.62 |
| 10:38 ET | 100 | 0.619 |
| 10:45 ET | 100 | 0.62 |
| 10:49 ET | 100 | 0.62 |
| 10:51 ET | 500 | 0.62 |
| 11:02 ET | 100 | 0.619 |
| 11:03 ET | 18750 | 0.617 |
| 11:05 ET | 22462 | 0.62 |
| 11:18 ET | 2000 | 0.6269 |
| 11:23 ET | 100 | 0.615 |
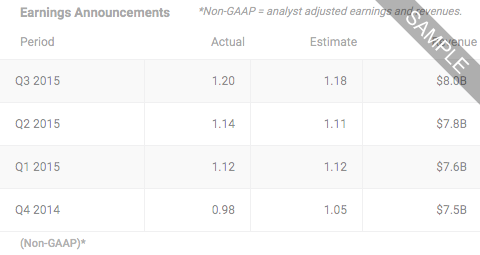
Oct 24, 2024
| Company sortable | Market Cap sortable | P/E Ratio (TTM) sortable | EPS Growth (5yr) sortable |
|---|---|---|---|
Passage Bio Inc | 37.3M | -0.5x | --- |
NextCure Inc | 36.6M | -0.6x | --- |
Surrozen Inc | 38.1M | -0.5x | --- |
HST Global Inc | 35.7M | -43.6x | --- |
Lantern Pharma Inc | 35.3M | -2.0x | --- |
Aeon Biopharma Inc | 34.7M | -0.1x | --- |
Company Information
Passage Bio, Inc. is a clinical-stage genetic medicines company focused on improving the lives of patients with neurodegenerative diseases. Its clinical product candidate, PBFT02, seeks to elevate progranulin levels to restore lysosomal function and slow disease progression across a variety of neurodegenerative diseases. PBFT02 utilizes an adeno-associated virus capsid to deliver a functional granulin gene, or GRN, encoding progranulin to the brain via intra cisterna magna administration. The lead indication for PBFT02 is frontotemporal dementia, or FTD, caused by progranulin deficiency, or FTD-GRN. Its development programs consist of PBFT02 for the treatment of FTD-GRN, PBFT02 for the treatment of FTD-C9orf72 and amyotrophic lateral sclerosis, and PBFT02 for the treatment of Alzheimer’s disease. Its clinical product candidates include PBGM01, PBKR03 and PBML04. It has one unnamed preclinical research program that explores multiple potential treatment targets for Huntington's disease.
Contact Information
- Headquarters
- One Commerce Square 2005 Market Street, 39Th FloorPHILADELPHIA, PA, United States 19103
- Phone
- 267-866-0312
- Fax
- 302-655-5049
Executives
- Interim Executive Chairwoman of the Board
- Maxine Gowen
- President, Chief Executive Officer, Director
- William Chou
- Chief Financial Officer
- Kathleen Borthwick
- Chief Scientific Officer
- Susan Browne
- General Counsel, Company Secretary
- Edgar Cale
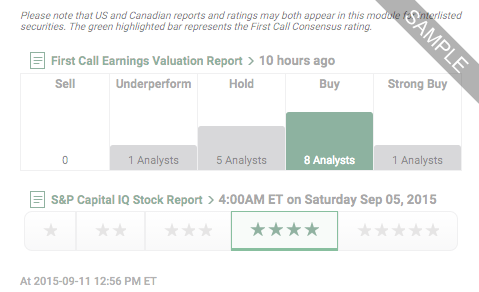
Our Ratings feature offers company-specific research ratings from providers such as First Call, S&P and Argus.
Open a New Account, or Login if you're a client.
You have access to a comprehensive selection of independent research reports from providers such as TD Securities, S&P, INK, and Argus.
Open a New Account, or Login if you're a client.
Market Cap | $37.3M |
|---|---|
Revenue (TTM) | $0.00 |
Shares Outstanding | 61.8M |
Dividend Yield | 0.00% |
Annual Dividend Rate | --- |
Ex-Dividend Date | 01-01-01 |
Pay Date | 01-01-01 |
Beta | 1.19 |
EPS | $-1.36 |
Book Value | $2.03 |
P/E Ratio | -0.5x |
Price/Sales (TTM) | --- |
Price/Cash Flow (TTM) | --- |
Operating Margin | --- |
Our Calendar feature allows you to view a wide selection of market and company events, including earnings, dividends, splits, rating changes, guidance, and more. Access past, current and future events in WebBroker.
Open a New Account, or Login if you're a client.