Spruce Biosciences IncNASDAQ:SPRBNASDAQ FSI:D. Deficient (Issuer failed to meet continued listing requirements)
- LAST PRICE0.5151
- TODAY'S CHANGE (%)0.0029 (0.5662%)
- Bid / Lots0.5123/ 5
- Ask / Lots0.5177/ 8
- Open / Previous Close0.5120 / 0.5122
- Day Range
- 52 Week Range
- Volume137,956
Search Criteria
Filter search criteria using below inputs
Click on magnifying glass icon to search
| Company | Country | Symbol |
|---|
- TSX Comp
- TSX Venture
- DJIA
- S&P 500
- NASDAQ

| Time | Volume | SPRB |
|---|---|---|
| 09:32 ET | 21835 | 0.520499 |
| 09:33 ET | 24900 | 0.524 |
| 09:35 ET | 3000 | 0.521 |
| 09:37 ET | 2000 | 0.53 |
| 09:51 ET | 126 | 0.5297 |
| 09:55 ET | 1169 | 0.5121 |
| 09:57 ET | 150 | 0.5232 |
| 10:02 ET | 10256 | 0.512 |
| 10:06 ET | 200 | 0.5201 |
| 10:27 ET | 100 | 0.5248 |
| 10:45 ET | 577 | 0.5121 |
| 10:51 ET | 40172 | 0.5121 |
| 11:07 ET | 8000 | 0.5123 |
| 11:39 ET | 500 | 0.522 |
| 11:52 ET | 1000 | 0.52 |
| 12:08 ET | 322 | 0.5219 |
| 12:24 ET | 481 | 0.515 |
| 12:33 ET | 20557 | 0.5123 |
| 12:35 ET | 100 | 0.5151 |
| 12:53 ET | 100 | 0.5151 |
| 12:57 ET | 100 | 0.5151 |
| 01:26 ET | 1340 | 0.5151 |
| 01:36 ET | 100 | 0.5151 |
Nov 12, 2024
DJ Spruce Biosciences Price Target Maintained With a $2.00/Share by RBC Capital, from 10:05AM ET on Tuesday Nov 12, 2024 by Dow Jones
10:05AM ET on Tuesday Nov 12, 2024 by Dow Jones
Nov 11, 2024
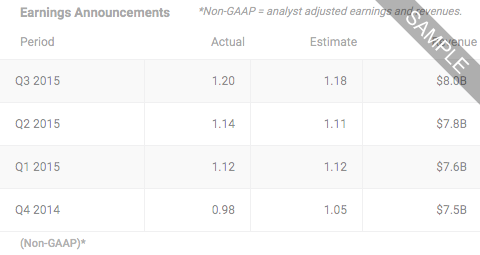
*DJ Spruce Biosciences 3Q Loss/Shr 21c >SPRB, from 8:00AM ET on Monday Nov 11, 2024 by Dow Jones
8:00AM ET on Monday Nov 11, 2024 by Dow JonesSpruce Biosciences Reports Third Quarter 2024 Financial Results and Provides Corporate Updates, from 8:00AM ET on Monday Nov 11, 2024 by Dow Jones
8:00AM ET on Monday Nov 11, 2024 by Dow Jones
Nov 4, 2024
Nov 3, 2024
Investors Are Invited To Join The Schall Law Firm In An Inquiry Into Spruce Biosciences Inc For Securities Law Violations, from 11:01AM ET on Sunday Nov 03, 2024 by Dow Jones
11:01AM ET on Sunday Nov 03, 2024 by Dow Jones
| Company sortable | Market Cap sortable | P/E Ratio (TTM) sortable | EPS Growth (5yr) sortable |
|---|---|---|---|
Spruce Biosciences Inc | 20.0M | -0.5x | --- |
Jaguar Health Inc | 9.6M | -0.1x | --- |
Syros Pharmaceuticals Inc | 7.9M | -0.1x | --- |
Aspira Women's Health Inc | 12.0M | -0.6x | --- |
Curis Inc | 33.5M | -0.5x | --- |
Bolt Biotherapeutics Inc | 22.1M | -0.4x | --- |
Company Information
Spruce Biosciences, Inc. is a late-stage biopharmaceutical company focused on developing and commercializing therapies for rare endocrine disorders with significant unmet medical needs. The Company is initially developing its wholly owned product candidate, Tildacerfont, as the potential first non-steroidal therapy for patients suffering from classic congenital adrenal hyperplasia (CAH). It is also developing Tildacerfont for females suffering from polycystic ovary syndrome (PCOS). It has initiated CAHmelia-203, a placebo-controlled, double-blind Phase IIb clinical trial in adult patients with classic CAH with elevated levels of androstenedione (A4) at baseline. It has also initiated CAHmelia-204, a second Phase IIb clinical trial in adult patients with classic CAH on supraphysiologic doses of glucocorticoids with normal or near normal levels of A4 at baseline focused on glucocorticoid reduction. The Company also investigates tildacerfont for the treatment of classic CAH in children.
Contact Information
- Headquarters
- 611 Gateway Boulevard, Suite 740SOUTH SAN FRANCISCO, CA, United States 94080
- Phone
- 415-655-4168
- Fax
- 302-636-5454
Executives
- Executive Chairman of the Board
- Michael Grey
- President, Chief Financial Officer
- Samir Gharib
- Chief Executive Officer, Director
- Javier Szwarcberg
- Chief Medical Officer
- Ralph Charlton
- Independent Director
- Tiba Aynechi
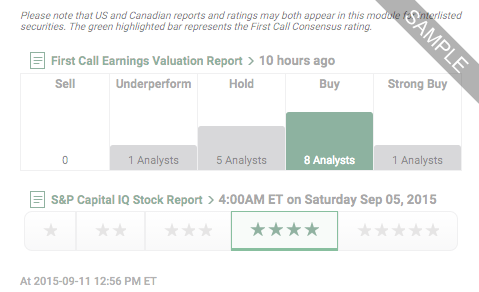
Our Ratings feature offers company-specific research ratings from providers such as First Call, S&P and Argus.
Open a New Account, or Login if you're a client.
You have access to a comprehensive selection of independent research reports from providers such as TD Securities, S&P, INK, and Argus.
Open a New Account, or Login if you're a client.
Market Cap | $20.0M |
|---|---|
Revenue (TTM) | $7.1M |
Shares Outstanding | 41.3M |
Dividend Yield | 0.00% |
Annual Dividend Rate | --- |
Ex-Dividend Date | 01-01-01 |
Pay Date | 01-01-01 |
Beta | 2.33 |
EPS | $-0.96 |
Book Value | $1.86 |
P/E Ratio | -0.5x |
Price/Sales (TTM) | 2.8 |
Price/Cash Flow (TTM) | --- |
Operating Margin | -608.94% |
Our Calendar feature allows you to view a wide selection of market and company events, including earnings, dividends, splits, rating changes, guidance, and more. Access past, current and future events in WebBroker.
Open a New Account, or Login if you're a client.