Kezar Life Sciences IncNASDAQ:KZRNASDAQ FSI:D. Deficient (Issuer failed to meet continued listing requirements)
- LAST PRICE0.6820
- TODAY'S CHANGE (%)-0.0122 (-1.7574%)
- Bid / Lots0.6820/ 1
- Ask / Lots0.7003/ 5
- Open / Previous Close0.6905 / 0.6942
- Day Range
- 52 Week Range
- Volume9,677
Search Criteria
Filter search criteria using below inputs
Click on magnifying glass icon to search
| Company | Country | Symbol |
|---|
- TSX Comp
- TSX Venture
- DJIA
- S&P 500
- NASDAQ

| Time | Volume | KZR |
|---|---|---|
| 09:32 ET | 1331 | 0.6905 |
| 09:36 ET | 300 | 0.6942 |
| 09:41 ET | 1099 | 0.6938 |
| 09:45 ET | 100 | 0.678 |
| 09:48 ET | 400 | 0.68 |
| 09:50 ET | 370 | 0.6801 |
| 09:52 ET | 200 | 0.68 |
| 09:56 ET | 200 | 0.681 |
| 09:57 ET | 100 | 0.681 |
| 09:59 ET | 1453 | 0.68 |
| 10:01 ET | 300 | 0.68 |
| 10:03 ET | 300 | 0.6812 |
| 10:06 ET | 1700 | 0.6813 |
| 10:10 ET | 200 | 0.6815 |
| 10:12 ET | 400 | 0.682 |
Jun 7, 2024
Kezar Announces Inducement Grant Under NASDAQ Listing Rule 5635(c)(4), from 5:59PM ET on Friday Jun 07, 2024 by Dow Jones
5:59PM ET on Friday Jun 07, 2024 by Dow Jones
May 29, 2024
Kezar Life Sciences to Participate in the Jefferies Global Healthcare Conference, from 4:01PM ET on Wednesday May 29, 2024 by Dow Jones
4:01PM ET on Wednesday May 29, 2024 by Dow Jones
| Company sortable | Market Cap sortable | P/E Ratio (TTM) sortable | EPS Growth (5yr) sortable |
|---|---|---|---|
Kezar Life Sciences Inc | 51.7M | -0.5x | --- |
LAVA Therapeutics NV | 60.5M | -2.2x | --- |
DURECT Corp | 48.4M | -2.0x | --- |
BioLine RX Ltd | 54.9M | -0.9x | --- |
Curis Inc | 51.9M | -1.0x | --- |
Atara Biotherapeutics Inc | 65.6M | -0.2x | --- |
Company Information
Kezar Life Sciences, Inc. is a clinical-stage biopharmaceutical company focused on discovering and developing small molecule therapeutics to treat unmet needs in immune-mediated diseases and cancer. The Company's lead product candidate, zetomipzomib, is a first-in-class selective immunoproteasome inhibitor that has completed Phase Ia testing in healthy volunteers and a Phase Ib/IIa clinical trial in patients with systemic lupus erythematosus (SLE), with or without lupus nephritis (LN). Its oncology product candidate, KZR-261, is a small molecule agent, targeting the Sec61 translocon and protein secretion pathway, being studied in an open-label Phase I clinical trial designed to evaluate safety and tolerability, pharmacokinetics and pharmacodynamics, as well to explore preliminary anti-tumor activity. This study is being conducted in two parts: dose escalation in patients with locally advanced or metastatic solid malignancies, and dose expansion in patients with selected tumor types.
Contact Information
- Headquarters
- 4000 Shoreline Ct Ste 300SOUTH SAN FRANCISCO, CA, United States 94080-2005
- Phone
- 650-822-5600
- Fax
- 302-636-5454
Executives
- Independent Chairman of the Board
- Graham Cooper
- Director
- Christopher Kirk
- Chief Financial Officer
- Marc Belsky
- Chief Business Officer
- Nick Mordwinkin
- Chief Legal Officer
- Mark Schiller
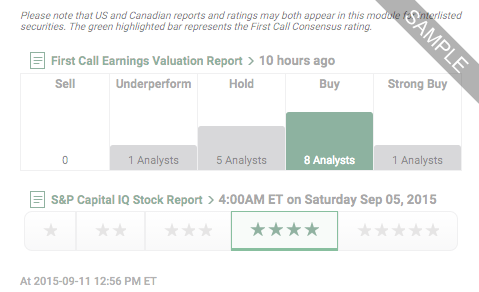
Our Ratings feature offers company-specific research ratings from providers such as First Call, S&P and Argus.
Open a New Account, or Login if you're a client.
You have access to a comprehensive selection of independent research reports from providers such as TD Securities, S&P, INK, and Argus.
Open a New Account, or Login if you're a client.
Market Cap | $51.7M |
|---|---|
Revenue (TTM) | $7.0M |
Shares Outstanding | 72.8M |
Dividend Yield | 0.00% |
Annual Dividend Rate | --- |
Ex-Dividend Date | 01-01-01 |
Pay Date | 01-01-01 |
Beta | 0.29 |
EPS | $-1.40 |
Book Value | $2.58 |
P/E Ratio | -0.5x |
Price/Sales (TTM) | 7.4 |
Price/Cash Flow (TTM) | --- |
Operating Margin | -1,591.77% |
Our Calendar feature allows you to view a wide selection of market and company events, including earnings, dividends, splits, rating changes, guidance, and more. Access past, current and future events in WebBroker.
Open a New Account, or Login if you're a client.