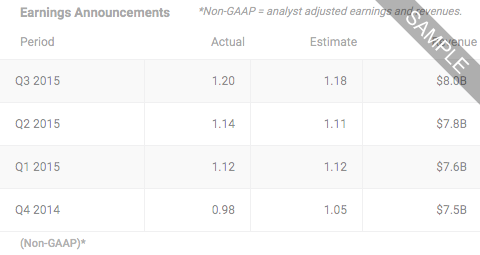
Taysha Gene Therapies IncNASDAQ:TSHA
- LAST PRICE2.8500
- TODAY'S CHANGE (%)-0.0400 (-1.3841%)
- Bid / Lots2.8500/ 36
- Ask / Lots2.8600/ 20
- Open / Previous Close2.8700 / 2.8900
- Day Range
- 52 Week Range
- Volume422,308
Search Criteria
Filter search criteria using below inputs
Click on magnifying glass icon to search
| Company | Country | Symbol |
|---|
- TSX Comp
- TSX Venture
- DJIA
- S&P 500
- NASDAQ

| Time | Volume | TSHA |
|---|---|---|
| 09:32 ET | 59613 | 2.89 |
| 09:33 ET | 62857 | 2.925 |
| 09:35 ET | 20376 | 2.915 |
| 09:37 ET | 12236 | 2.895 |
| 09:39 ET | 4600 | 2.87 |
| 09:42 ET | 11264 | 2.85 |
| 09:44 ET | 25576 | 2.85 |
| 09:46 ET | 26444 | 2.855 |
| 09:48 ET | 4318 | 2.87 |
| 09:50 ET | 34570 | 2.855 |
| 09:51 ET | 19320 | 2.85 |
| 09:53 ET | 12505 | 2.84 |
| 09:55 ET | 5016 | 2.87 |
| 09:57 ET | 11449 | 2.855 |
| 10:00 ET | 26341 | 2.87 |
| 10:02 ET | 34428 | 2.85 |
Nov 15, 2024
DJ Taysha Gene Therapies Is Maintained at Buy by Canaccord Genuity, from 9:41AM ET on Friday Nov 15, 2024 by Dow Jones
9:41AM ET on Friday Nov 15, 2024 by Dow Jones
Nov 14, 2024
DJ Taysha Gene Therapies Is Maintained at Buy by Chardan Capital, from 5:51PM ET on Thursday Nov 14, 2024 by Dow Jones
5:51PM ET on Thursday Nov 14, 2024 by Dow JonesDJ Taysha Gene Therapies Price Target Maintained With a $6.00/Share by Needham, from 1:54PM ET on Thursday Nov 14, 2024 by Dow Jones
1:54PM ET on Thursday Nov 14, 2024 by Dow JonesDJ Taysha Gene Therapies Price Target Maintained With a $7.00/Share by Cantor Fitzgerald, from 9:11AM ET on Thursday Nov 14, 2024 by Dow Jones
9:11AM ET on Thursday Nov 14, 2024 by Dow Jones
Nov 13, 2024
Taysha Gene Therapies Reports Third Quarter 2024 Financial Results and Provides Corporate Update, from 4:01PM ET on Wednesday Nov 13, 2024 by Dow Jones
4:01PM ET on Wednesday Nov 13, 2024 by Dow Jones
| Company sortable | Market Cap sortable | P/E Ratio (TTM) sortable | EPS Growth (5yr) sortable |
|---|---|---|---|
Taysha Gene Therapies Inc | 506.2M | -45.6x | --- |
MeiraGTx Holdings PLC | 463.4M | -4.9x | --- |
PureTech Health PLC | 515.9M | -7.3x | --- |
Cartesian Therapeutics Inc | 479.6M | -0.5x | --- |
Olema Pharmaceuticals Inc | 503.1M | -3.9x | --- |
Kalvista Pharmaceuticals Inc | 498.2M | -2.8x | --- |
Company Information
Taysha Gene Therapies Inc is a clinical-stage biotechnology company, which is focused on advancing adeno-associated virus (AAV)-based gene therapies for severe monogenic diseases of the central nervous system. The Company’s lead clinical program, TSHA-102, is in development for the treatment of Rett syndrome, a rare neurodevelopmental disorder. The Company is evaluating TSHA-102 in the REVEALPhase I/II adolescent and adult clinical trial, which is a first-in-human, open-label, randomized, dose escalation and dose-expansion, multicenter study evaluating the safety and preliminary efficacy of TSHA-102 in female patients aged 12-years and older with Rett syndrome. It has acquired a worldwide right to a clinical-stage, intrathecally dosed AAV9 gene therapy program, TSHA-120, for the treatment of giant axonal neuropathy (GAN). It received orphan drug designation from the European Commission for TSHA-120 for the treatment of GAN.
Contact Information
- Headquarters
- 3000 Pegasus Park Drive, Suite 1430DALLAS, TX, United States 75247
- Phone
- 214-612-0000
- Fax
- ---
Executives
- Chairman of the Board, Chief Executive Officer
- Sean Nolan
- President, Head of Research and Development, Director
- Sukumar Nagendran
- Chief Financial Officer
- Kamran Alam
- Director
- Alison Long
- Independent Director
- Phillip Donenberg
Our Ratings feature offers company-specific research ratings from providers such as First Call, S&P and Argus.
Open a New Account, or Login if you're a client.
You have access to a comprehensive selection of independent research reports from providers such as TD Securities, S&P, INK, and Argus.
Open a New Account, or Login if you're a client.
Market Cap | $506.2M |
|---|---|
Revenue (TTM) | $9.9M |
Shares Outstanding | 204.9M |
Dividend Yield | 0.00% |
Annual Dividend Rate | --- |
Ex-Dividend Date | 01-01-01 |
Pay Date | 01-01-01 |
Beta | 0.64 |
EPS | $-0.06 |
Book Value | $0.40 |
P/E Ratio | -45.6x |
Price/Sales (TTM) | 51.1 |
Price/Cash Flow (TTM) | --- |
Operating Margin | -899.35% |
Our Calendar feature allows you to view a wide selection of market and company events, including earnings, dividends, splits, rating changes, guidance, and more. Access past, current and future events in WebBroker.
Open a New Account, or Login if you're a client.