enVVeno Medical CorpNASDAQ:NVNO
- LAST PRICE3.4100
- TODAY'S CHANGE (%)0.0000 (0.0000%)
- Bid / Lots3.4000/ 3
- Ask / Lots3.4200/ 12
- Open / Previous Close3.4300 / 3.4100
- Day Range
- 52 Week Range
- Volume90,648
Search Criteria
Filter search criteria using below inputs
Click on magnifying glass icon to search
| Company | Country | Symbol |
|---|
- TSX Comp
- TSX Venture
- DJIA
- S&P 500
- NASDAQ

| Time | Volume | NVNO |
|---|---|---|
| 09:32 ET | 1275 | 3.44 |
| 09:33 ET | 2144 | 3.4 |
| 09:37 ET | 100 | 3.435 |
| 09:50 ET | 2130 | 3.41 |
| 09:51 ET | 244 | 3.42 |
| 09:53 ET | 740 | 3.42 |
| 09:55 ET | 2097 | 3.4 |
| 09:57 ET | 325 | 3.4035 |
| 10:04 ET | 1950 | 3.3612 |
| 10:06 ET | 200 | 3.385 |
| 10:11 ET | 100 | 3.385 |
| 10:22 ET | 250 | 3.39 |
| 10:38 ET | 212 | 3.41 |
| 10:40 ET | 145 | 3.41 |
| 10:44 ET | 200 | 3.41 |
| 10:45 ET | 2505 | 3.41 |
| 10:47 ET | 8312 | 3.42 |
| 10:49 ET | 5982 | 3.43 |
| 10:51 ET | 300 | 3.435 |
| 10:58 ET | 120 | 3.4275 |
| 11:02 ET | 703 | 3.42 |
| 11:03 ET | 200 | 3.415 |
| 11:05 ET | 300 | 3.42 |
| 11:30 ET | 2250 | 3.4101 |
| 11:34 ET | 7175 | 3.41 |
| 11:36 ET | 500 | 3.41 |
| 11:38 ET | 1758 | 3.41 |
| 11:45 ET | 360 | 3.43 |
| 11:52 ET | 100 | 3.415 |
| 11:54 ET | 600 | 3.415 |
| 11:56 ET | 1300 | 3.415 |
| 12:01 ET | 100 | 3.415 |
| 12:03 ET | 2100 | 3.42 |
| 12:17 ET | 400 | 3.425 |
| 12:19 ET | 390 | 3.425 |
| 12:24 ET | 200 | 3.41 |
| 12:30 ET | 100 | 3.41 |
| 12:32 ET | 1000 | 3.425 |
| 12:37 ET | 100 | 3.4377 |
| 12:39 ET | 300 | 3.4329 |
| 12:46 ET | 1252 | 3.42 |
| 12:48 ET | 200 | 3.42 |
| 01:04 ET | 393 | 3.44 |
| 01:09 ET | 720 | 3.435 |
| 01:13 ET | 300 | 3.4215 |
| 01:20 ET | 100 | 3.43 |
| 01:22 ET | 100 | 3.43 |
| 01:24 ET | 100 | 3.43 |
| 01:26 ET | 608 | 3.43 |
| 01:27 ET | 488 | 3.43 |
| 01:42 ET | 200 | 3.435 |
| 01:44 ET | 1500 | 3.4335 |
| 01:47 ET | 3100 | 3.435 |
| 01:51 ET | 200 | 3.43 |
| 01:56 ET | 404 | 3.43 |
| 01:58 ET | 400 | 3.42 |
| 02:03 ET | 700 | 3.41 |
| 02:18 ET | 8983 | 3.38 |
| 02:25 ET | 300 | 3.3888 |
| 02:32 ET | 100 | 3.39 |
| 02:36 ET | 200 | 3.39 |
| 02:38 ET | 1666 | 3.4 |
| 02:45 ET | 100 | 3.405 |
| 02:56 ET | 100 | 3.415 |
| 03:10 ET | 112 | 3.41 |
| 03:15 ET | 3080 | 3.42 |
| 03:19 ET | 404 | 3.435 |
| 03:21 ET | 600 | 3.42 |
| 03:24 ET | 300 | 3.42 |
| 03:30 ET | 495 | 3.42 |
| 03:32 ET | 500 | 3.41 |
| 03:33 ET | 2679 | 3.41 |
| 03:37 ET | 583 | 3.4188 |
| 03:42 ET | 1875 | 3.417572 |
| 03:44 ET | 700 | 3.41 |
| 03:46 ET | 100 | 3.41 |
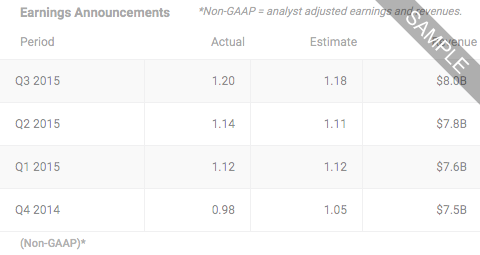
Nov 20, 2024
enVVeno Medical to Present Definitive One Year Data from the VenoValve U.S. Pivotal Trial Today at the 51st Annual VEITH Symposium, from 8:31AM ET on Wednesday Nov 20, 2024 by Dow Jones
8:31AM ET on Wednesday Nov 20, 2024 by Dow Jones
Nov 19, 2024
enVVeno Medical Submits the VenoValve PMA Application Seeking FDA Approval, from 8:15AM ET on Tuesday Nov 19, 2024 by Dow Jones
8:15AM ET on Tuesday Nov 19, 2024 by Dow Jones
Oct 31, 2024
enVVeno Medical Reports Third Quarter 2024 Financial Results and Confirms Guidance for Release of Definitive VenoValve Data in November and Completion of FDA Premarket Approval Application for VenoValve in Q4 of this Year, from 8:59AM ET on Thursday Oct 31, 2024 by Dow Jones
8:59AM ET on Thursday Oct 31, 2024 by Dow Jones
Oct 28, 2024
enVVeno Medical Successfully Initiates 6-Month Pre-Clinical GLP Study Evaluating enVVe(R), from 8:31AM ET on Monday Oct 28, 2024 by Dow Jones
8:31AM ET on Monday Oct 28, 2024 by Dow Jones
| Company sortable | Market Cap sortable | P/E Ratio (TTM) sortable | EPS Growth (5yr) sortable |
|---|---|---|---|
enVVeno Medical Corp | 59.8M | -2.6x | --- |
Citius Oncology Inc | 60.8M | 3.5x | --- |
Entera Bio Ltd | 58.6M | -6.3x | --- |
Immuneering Corp | 62.3M | -1.1x | --- |
Mural Oncology PLC | 57.2M | -0.4x | --- |
X4 Pharmaceuticals Inc | 57.0M | -4.0x | --- |
Company Information
enVVeno Medical Corporation is a late-stage clinical med-tech company. The Company is focused on the advancement of bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of venous disease. The Company is developing surgical and non-surgical replacement venous valves for patients suffering from severe Chronic Venous Insufficiency (CVI) of the deep venous system of the leg. The Company's lead product, VenoValve, which is a porcine based replacement venous valve developed to surgically implanted in the deep venous system of the leg to treat severe CVI. The VenoValve is implanted into the femoral vein of the patient in an open surgical procedure via a five-to-six-inch incision in the upper thigh. The VenoValve is being evaluated in the surgical anti-reflux venous valve endoprosthesis (SAVVE) United States clinical trial. The Company is also developing a second product called enVVe, which is a non-surgical, transcatheter based replacement venous valve.
Contact Information
- Headquarters
- 70 DopplerIRVINE, CA, United States 92618-4306
- Phone
- 949-261-2900
- Fax
- 949-261-2992
Executives
- Chief Executive Officer, Director
- Robert Berman
- Chief Financial Officer, Treasurer
- Craig Glynn
- Senior Vice President, Chief Technology Officer
- Hamed Alavi
- Senior Vice President, Chief Medical Officer
- Marc Glickman
- Chief Commercial Officer
- Andrew Cormack
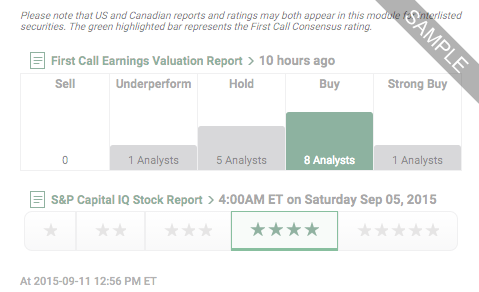
Our Ratings feature offers company-specific research ratings from providers such as First Call, S&P and Argus.
Open a New Account, or Login if you're a client.
You have access to a comprehensive selection of independent research reports from providers such as TD Securities, S&P, INK, and Argus.
Open a New Account, or Login if you're a client.
Market Cap | $59.8M |
|---|---|
Revenue (TTM) | $0.00 |
Shares Outstanding | 17.5M |
Dividend Yield | 0.00% |
Annual Dividend Rate | --- |
Ex-Dividend Date | 01-01-01 |
Pay Date | 01-01-01 |
Beta | 1.25 |
EPS | $-1.33 |
Book Value | $3.47 |
P/E Ratio | -2.6x |
Price/Sales (TTM) | --- |
Price/Cash Flow (TTM) | --- |
Operating Margin | --- |
Our Calendar feature allows you to view a wide selection of market and company events, including earnings, dividends, splits, rating changes, guidance, and more. Access past, current and future events in WebBroker.
Open a New Account, or Login if you're a client.