Atea Pharmaceuticals IncNASDAQ:AVIR
- LAST PRICE3.9250
- TODAY'S CHANGE (%)0.2250 (6.0811%)
- Bid / Lots3.9200/ 6
- Ask / Lots3.9300/ 3
- Open / Previous Close3.7300 / 3.7000
- Day Range
- 52 Week Range
- Volume117,119
Search Criteria
Filter search criteria using below inputs
Click on magnifying glass icon to search
| Company | Country | Symbol |
|---|
- TSX Comp
- TSX Venture
- DJIA
- S&P 500
- NASDAQ

| Time | Volume | AVIR |
|---|---|---|
| 09:32 ET | 1770 | 3.73 |
| 09:34 ET | 4631 | 3.74 |
| 09:36 ET | 200 | 3.77 |
| 09:39 ET | 1045 | 3.8 |
| 09:41 ET | 2700 | 3.805 |
| 09:43 ET | 6034 | 3.775 |
| 09:45 ET | 2284 | 3.8 |
| 09:52 ET | 22731 | 3.83 |
| 09:54 ET | 28019 | 3.865 |
| 09:56 ET | 200 | 3.87 |
| 09:57 ET | 304 | 3.88 |
| 09:59 ET | 22868 | 3.91 |
| 10:01 ET | 3322 | 3.91 |
| 10:03 ET | 2062 | 3.91 |
| 10:06 ET | 100 | 3.915 |
| 10:08 ET | 3229 | 3.9202 |
| 10:10 ET | 139 | 3.9204 |
| 10:12 ET | 7323 | 3.925 |
| 10:14 ET | 1224 | 3.925 |
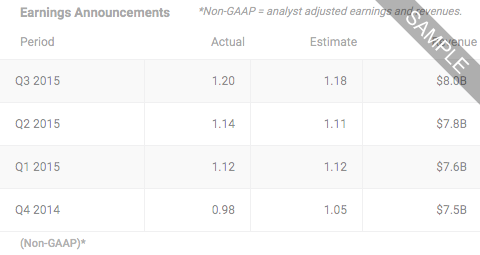
Apr 29, 2024
Atea Announces Presentation of Data Highlighting Favorable Safety Profile of Bemnifosbuvir at ESCMID Global 2024, from 7:00AM ET on Monday Apr 29, 2024 by Dow Jones
7:00AM ET on Monday Apr 29, 2024 by Dow Jones
| Company sortable | Market Cap sortable | P/E Ratio (TTM) sortable | EPS Growth (5yr) sortable |
|---|---|---|---|
Atea Pharmaceuticals Inc | 311.4M | -2.4x | --- |
Fibrobiologics Inc | 308.5M | -10.4x | --- |
Nautilus Biotechnology Inc | 317.7M | -4.9x | --- |
Rani Therapeutics Holdings Inc | 321.6M | -4.7x | --- |
Anavex Life Sciences Corp | 299.7M | -6.8x | --- |
Lyra Therapeutics Inc | 308.3M | -4.0x | --- |
Company Information
Atea Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company. The Company is focused on discovering, developing, and commercializing antiviral therapeutics to improve the lives of patients suffering from serious viral infections. The Company is engaged in is conducting a Phase 3 clinical trial evaluating bemnifosbuvir for the treatment of Coronavirus disease 2019 (COVID-19). It is also conducting a Phase 2 clinical trial evaluating the combination of bemnifosbuvir and ruzasvir for the treatment of hepatitis C virus (HCV). The bemnifosbuvir (AT-527), is an investigational, novel, orally administered guanosine nucleotide analog polymerase inhibitor that combines a unique nucleotide scaffold with novel double prodrugs for the intended purpose of inhibiting the enzymes central to viral replication. SUNRISE-3 is designed to evaluate bemnifosbuvir as monotherapy, but it is also exploring the effect of combination therapy in a smaller sub-set of patients.
Contact Information
- Headquarters
- 225 Franklin Street, Suite 2100BOSTON, MA, United States 02110
- Phone
- 857-204-8109
- Fax
- 302-655-5049
Executives
- Chairman of the Board, President, Chief Executive Officer, Founder
- Jean-Pierre Sommadossi
- Chief Financial Officer, Executive Vice President - Legal , Secretary
- Andrea Corcoran
- Executive Vice President, Chief Accounting Officer
- Wayne Foster
- Chief Development Officer
- Janet Hammond
- Chief Medical Officer
- Maria Horga
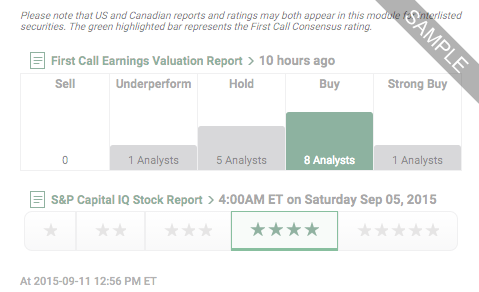
Our Ratings feature offers company-specific research ratings from providers such as First Call, S&P and Argus.
Open a New Account, or Login if you're a client.
You have access to a comprehensive selection of independent research reports from providers such as TD Securities, S&P, INK, and Argus.
Open a New Account, or Login if you're a client.
Market Cap | $311.4M |
|---|---|
Revenue (TTM) | $0.00 |
Shares Outstanding | 84.2M |
Dividend Yield | 0.00% |
Annual Dividend Rate | --- |
Ex-Dividend Date | 01-01-01 |
Pay Date | 01-01-01 |
Beta | 0.19 |
EPS | $-1.63 |
Book Value | $6.65 |
P/E Ratio | -2.4x |
Price/Sales (TTM) | --- |
Price/Cash Flow (TTM) | --- |
Operating Margin | --- |
Our Calendar feature allows you to view a wide selection of market and company events, including earnings, dividends, splits, rating changes, guidance, and more. Access past, current and future events in WebBroker.
Open a New Account, or Login if you're a client.